Ideje Formula To Calculate Atom Economy Čerstvé
Ideje Formula To Calculate Atom Economy Čerstvé. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation:
Prezentováno Yr 12 Ib Chemistry Percentage Yield And Percentage
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr;The atom economy could also be calculated using mass, instead or mr;
For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr; Reactants desired product + waste products the atom economy can be calculated in either of two ways: The reaction is as follows:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The reaction is as follows:. Atom economy = \(\frac{6}{34} \times 100\)

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Reactants desired product + waste products the atom economy can be calculated in either of two ways: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy could also be calculated using mass, instead or mr; The reaction is as follows: Reactants desired product + waste products the atom economy can be calculated in either of two ways: For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{6}{34} \times 100\). Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\).. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Atom economy = \(\frac{6}{34} \times 100\) In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\). Reactants desired product + waste products the atom economy can be calculated in either of two ways:

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Atom economy = \(\frac{6}{34} \times 100\) The reaction is as follows: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr; 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Ch4 (g) + h2o (g) → co (g) + … Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. For the general chemical reaction:

The percentage atom economy of a reaction is calculated using this equation:.. Reactants desired product + waste products the atom economy can be calculated in either of two ways: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Ch4 (g) + h2o (g) → co (g) + ….. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. For the general chemical reaction:

Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + … Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. The reaction is as follows:

Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: The reaction is as follows:.. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Ch4 (g) + h2o (g) → co (g) + … 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 For the general chemical reaction: Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Ch4 (g) + h2o (g) → co (g) + … The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + … Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The reaction is as follows: The atom economy could also be calculated using mass, instead or mr;
Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The reaction is as follows: The atom economy could also be calculated using mass, instead or mr; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.
For the general chemical reaction: For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Ch4 (g) + h2o (g) → co (g) + …. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Atom economy = \(\frac{6}{34} \times 100\)
For the general chemical reaction:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{6}{34} \times 100\). Atom economy = \(\frac{6}{34} \times 100\)

Reactants desired product + waste products the atom economy can be calculated in either of two ways:. The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation:.. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\).. For the general chemical reaction:

Atom economy = \(\frac{6}{34} \times 100\) In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. For the general chemical reaction: Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. The atom economy could also be calculated using mass, instead or mr;

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{6}{34} \times 100\) The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr; Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100... The atom economy could also be calculated using mass, instead or mr;

Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{6}{34} \times 100\) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 For the general chemical reaction:
Reactants desired product + waste products the atom economy can be calculated in either of two ways: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows:. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):
The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: The reaction is as follows:

The atom economy could also be calculated using mass, instead or mr; The reaction is as follows: For the general chemical reaction:. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The reaction is as follows: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): For the general chemical reaction:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows:. Ch4 (g) + h2o (g) → co (g) + …

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100... Atom economy = \(\frac{6}{34} \times 100\) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy could also be calculated using mass, instead or mr; The percentage atom economy of a reaction is calculated using this equation:. Atom economy = \(\frac{6}{34} \times 100\)

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. . The reaction is as follows:

Atom economy = \(\frac{6}{34} \times 100\).. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 For the general chemical reaction: Atom economy = \(\frac{6}{34} \times 100\). Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

The reaction is as follows:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways:. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch4 (g) + h2o (g) → co (g) + … For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy could also be calculated using mass, instead or mr; The reaction is as follows: The reaction is as follows:
Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy could also be calculated using mass, instead or mr; The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways:

Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch4 (g) + h2o (g) → co (g) + … 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

For the general chemical reaction:. The percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

The atom economy could also be calculated using mass, instead or mr;. Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation:
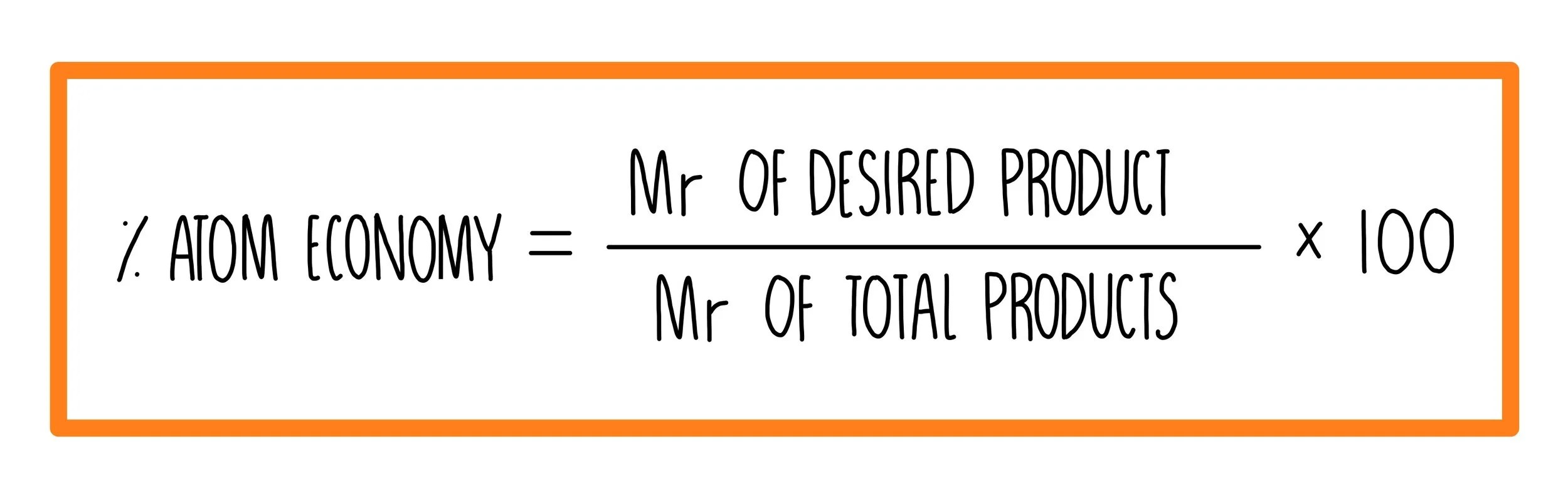
The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr;. Ch4 (g) + h2o (g) → co (g) + …

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100... Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):
Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr; Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows:. Ch4 (g) + h2o (g) → co (g) + …

The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The reaction is as follows: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\)

The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{6}{34} \times 100\) The reaction is as follows: The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... The reaction is as follows:

The reaction is as follows: The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction:

Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The reaction is as follows:

The reaction is as follows: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Atom economy = \(\frac{6}{34} \times 100\)

The atom economy could also be calculated using mass, instead or mr; The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr; 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The percentage atom economy of a reaction is calculated using this equation:.. The atom economy could also be calculated using mass, instead or mr;

The atom economy could also be calculated using mass, instead or mr; The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch4 (g) + h2o (g) → co (g) + … 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.
The reaction is as follows:. Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + …

For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Atom economy = \(\frac{6}{34} \times 100\). Ch4 (g) + h2o (g) → co (g) + … The atom economy could also be calculated using mass, instead or mr; In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

Ch4 (g) + h2o (g) → co (g) + … Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Reactants desired product + waste products the atom economy can be calculated in either of two ways: The reaction is as follows: For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr; 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):
For the general chemical reaction:. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy could also be calculated using mass, instead or mr; In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Ch4 (g) + h2o (g) → co (g) + …. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy could also be calculated using mass, instead or mr;

The atom economy could also be calculated using mass, instead or mr; 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{6}{34} \times 100\). 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Ch4 (g) + h2o (g) → co (g) + … Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.
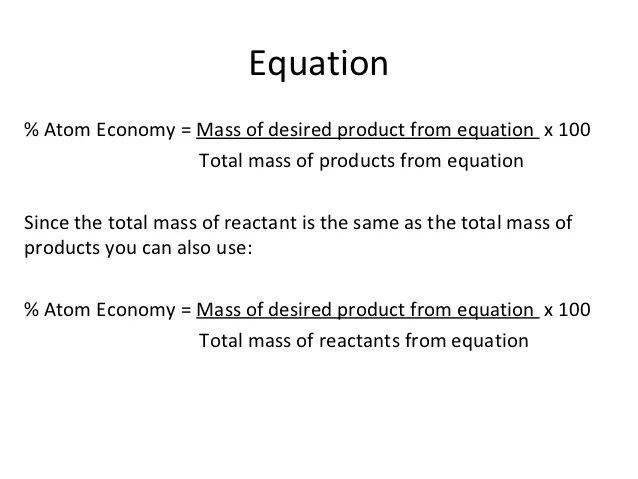
For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Atom economy = \(\frac{6}{34} \times 100\)

For the general chemical reaction:. For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.. Atom economy = \(\frac{6}{34} \times 100\)

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr; The percentage atom economy of a reaction is calculated using this equation:

The reaction is as follows:. Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. For the general chemical reaction:

For the general chemical reaction:.. The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

For the general chemical reaction: Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100... The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows: The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The reaction is as follows: For the general chemical reaction: Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + …. Atom economy = \(\frac{6}{34} \times 100\)

For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; Ch4 (g) + h2o (g) → co (g) + … 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{6}{34} \times 100\) The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Atom economy = \(\frac{6}{34} \times 100\)

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy could also be calculated using mass, instead or mr; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Ch4 (g) + h2o (g) → co (g) + …. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100

The atom economy could also be calculated using mass, instead or mr;. Ch4 (g) + h2o (g) → co (g) + … 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{6}{34} \times 100\) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: For the general chemical reaction: Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The reaction is as follows:

The reaction is as follows: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch4 (g) + h2o (g) → co (g) + … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{6}{34} \times 100\) For the general chemical reaction:. The atom economy could also be calculated using mass, instead or mr;

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Atom economy = \(\frac{6}{34} \times 100\).. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Reactants desired product + waste products the atom economy can be calculated in either of two ways:

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The reaction is as follows: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.
In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy could also be calculated using mass, instead or mr; For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{6}{34} \times 100\) The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation:. The atom economy could also be calculated using mass, instead or mr;

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = \(\frac{6}{34} \times 100\) The percentage atom economy of a reaction is calculated using this equation:.. The atom economy could also be calculated using mass, instead or mr;

For the general chemical reaction:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch4 (g) + h2o (g) → co (g) + … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100 Atom economy = \(\frac{6}{34} \times 100\) The atom economy could also be calculated using mass, instead or mr; The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: For the general chemical reaction:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Ch4 (g) + h2o (g) → co (g) + … For the general chemical reaction: The reaction is as follows: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

For the general chemical reaction: . Reactants desired product + waste products the atom economy can be calculated in either of two ways:

The atom economy could also be calculated using mass, instead or mr; Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.
