Kolekce 32 John Dalton Atom Diagram
Kolekce 32 John Dalton Atom Diagram. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.
Nejchladnější Daltons Atomic Theory John Dalton 1766 1844 Proposed
Diagram john dalton atomic model. Dalton's atomic model sets up the building blocks for others to improve on. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'.The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.
Diagram john dalton atomic model. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Part ii, 1810) was the first application of atomic theory to chemistry. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. His theory was based on two verified scientific laws: It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

Experimental foundation of atomic chemistry. Dalton based his theory on the law of conservation of mass and the law of constant composition.. The first part of his theory states that all matter is made of atoms, which are indivisible.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dalton based his theory on the law of conservation of mass and the law of constant composition. This became the basis as dalton's law (aka... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

This became the basis as dalton's law (aka. It stated that all matter was made up of small, indivisible particles known as 'atoms'. He defined an atom as the smallest indivisible particle. Dalton based his theory on the law of conservation of mass and the law of constant composition. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Part ii, 1810) was the first application of atomic theory to chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic model sets up the building blocks for others to improve on. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete... Experimental foundation of atomic chemistry.

The law of conservation of mass and the law of …. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

Experimental foundation of atomic chemistry. The law of conservation of mass and the law of … Diagram john dalton atomic model. He defined an atom as the smallest indivisible particle. It stated that all matter was made up of small, indivisible particles known as 'atoms'. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Though some of his conclusions were incorrect, his contributions were vital. Experimental foundation of atomic chemistry. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.

Diagram john dalton atomic model. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. This became the basis as dalton's law (aka. The first part of his theory states that all matter is made of atoms, which are indivisible. He defined an atom as the smallest indivisible particle.

His theory was based on two verified scientific laws: Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Part ii, 1810) was the first application of atomic theory to chemistry. His theory was based on two verified scientific laws: This became the basis as dalton's law (aka. Dalton's atomic model sets up the building blocks for others to improve on. The first part of his theory states that all matter is made of atoms, which are indivisible. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Dalton based his theory on the law of conservation of mass and the law of constant composition. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Part ii, 1810) was the first application of atomic theory to chemistry.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.. Dec 01, 2014 · a depiction of the atomic structure of the helium atom.

His book a new system of chemical philosophy ( part i, 1808; His book a new system of chemical philosophy ( part i, 1808; This became the basis as dalton's law (aka. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Experimental foundation of atomic chemistry. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's atomic model sets up the building blocks for others to improve on. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The first part of his theory states that all matter is made of atoms, which are indivisible. The law of conservation of mass and the law of …. Experimental foundation of atomic chemistry.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties... Diagram john dalton atomic model... Dalton based his theory on the law of conservation of mass and the law of constant composition.

Part ii, 1810) was the first application of atomic theory to chemistry... . It stated that all matter was made up of small, indivisible particles known as 'atoms'.

His book a new system of chemical philosophy ( part i, 1808;. Though some of his conclusions were incorrect, his contributions were vital. The first part of his theory states that all matter is made of atoms, which are indivisible.

Part ii, 1810) was the first application of atomic theory to chemistry. Though some of his conclusions were incorrect, his contributions were vital. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dec 01, 2014 · a depiction of the atomic structure of the helium atom.. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.

This became the basis as dalton's law (aka. Diagram john dalton atomic model. The law of conservation of mass and the law of … Dalton's atomic model sets up the building blocks for others to improve on. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dalton's atomic model sets up the building blocks for others to improve on. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. His theory was based on two verified scientific laws: Experimental foundation of atomic chemistry.. Dalton based his theory on the law of conservation of mass and the law of constant composition.

The first part of his theory states that all matter is made of atoms, which are indivisible. Experimental foundation of atomic chemistry. His book a new system of chemical philosophy ( part i, 1808;. Dalton's atomic model sets up the building blocks for others to improve on.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Part ii, 1810) was the first application of atomic theory to chemistry. Dalton's atomic model sets up the building blocks for others to improve on. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. This became the basis as dalton's law (aka. The law of conservation of mass and the law of … Dec 01, 2014 · a depiction of the atomic structure of the helium atom. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Part ii, 1810) was the first application of atomic theory to chemistry.

His book a new system of chemical philosophy ( part i, 1808;.. Dalton based his theory on the law of conservation of mass and the law of constant composition. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory... John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. The first part of his theory states that all matter is made of atoms, which are indivisible. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton based his theory on the law of conservation of mass and the law of constant composition. Experimental foundation of atomic chemistry. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dalton's atomic model sets up the building blocks for others to improve on.. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808.

This became the basis as dalton's law (aka... Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. The law of conservation of mass and the law of …

Dec 01, 2014 · a depiction of the atomic structure of the helium atom. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Dalton based his theory on the law of conservation of mass and the law of constant composition. The law of conservation of mass and the law of … The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Diagram john dalton atomic model. This became the basis as dalton's law (aka. His theory was based on two verified scientific laws: Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. It stated that all matter was made up of small, indivisible particles known as 'atoms'. The law of conservation of mass and the law of …

Part ii, 1810) was the first application of atomic theory to chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible.. Dalton's atomic model sets up the building blocks for others to improve on.

His theory was based on two verified scientific laws: Dalton's atomic model sets up the building blocks for others to improve on. His theory was based on two verified scientific laws: John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Though some of his conclusions were incorrect, his contributions were vital. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

Though some of his conclusions were incorrect, his contributions were vital.. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Though some of his conclusions were incorrect, his contributions were vital. His theory was based on two verified scientific laws: English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808.. Dec 01, 2014 · a depiction of the atomic structure of the helium atom.

Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.. The law of conservation of mass and the law of …

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dalton based his theory on the law of conservation of mass and the law of constant composition. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Part ii, 1810) was the first application of atomic theory to chemistry. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Diagram john dalton atomic model. Though some of his conclusions were incorrect, his contributions were vital. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom... Experimental foundation of atomic chemistry. Though some of his conclusions were incorrect, his contributions were vital. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. The first part of his theory states that all matter is made of atoms, which are indivisible. His book a new system of chemical philosophy ( part i, 1808; It stated that all matter was made up of small, indivisible particles known as 'atoms'. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time... He defined an atom as the smallest indivisible particle.

The first part of his theory states that all matter is made of atoms, which are indivisible. The first part of his theory states that all matter is made of atoms, which are indivisible. Part ii, 1810) was the first application of atomic theory to chemistry. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. His book a new system of chemical philosophy ( part i, 1808;

His theory was based on two verified scientific laws: Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Part ii, 1810) was the first application of atomic theory to chemistry. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'.

This became the basis as dalton's law (aka.. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. His theory was based on two verified scientific laws: John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The first part of his theory states that all matter is made of atoms, which are indivisible. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. Experimental foundation of atomic chemistry. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Part ii, 1810) was the first application of atomic theory to chemistry. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. His theory was based on two verified scientific laws: He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Experimental foundation of atomic chemistry... This became the basis as dalton's law (aka.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Experimental foundation of atomic chemistry. The law of conservation of mass and the law of … The first part of his theory states that all matter is made of atoms, which are indivisible.

The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory... Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. He defined an atom as the smallest indivisible particle.. Dalton based his theory on the law of conservation of mass and the law of constant composition.

Dalton based his theory on the law of conservation of mass and the law of constant composition. Experimental foundation of atomic chemistry. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. His book a new system of chemical philosophy ( part i, 1808;. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

Part ii, 1810) was the first application of atomic theory to chemistry... . His theory was based on two verified scientific laws:

His theory was based on two verified scientific laws: This became the basis as dalton's law (aka. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dalton's atomic model sets up the building blocks for others to improve on. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. The first part of his theory states that all matter is made of atoms, which are indivisible. Part ii, 1810) was the first application of atomic theory to chemistry. Experimental foundation of atomic chemistry. His theory was based on two verified scientific laws: English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Experimental foundation of atomic chemistry. The law of conservation of mass and the law of … Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. His book a new system of chemical philosophy ( part i, 1808; He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Experimental foundation of atomic chemistry.

Dalton based his theory on the law of conservation of mass and the law of constant composition.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. Though some of his conclusions were incorrect, his contributions were vital. Dalton based his theory on the law of conservation of mass and the law of constant composition. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. This became the basis as dalton's law (aka. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. His book a new system of chemical philosophy ( part i, 1808; It stated that all matter was made up of small, indivisible particles known as 'atoms'... Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. It stated that all matter was made up of small, indivisible particles known as 'atoms'. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though some of his conclusions were incorrect, his contributions were vital. His book a new system of chemical philosophy ( part i, 1808; Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Part ii, 1810) was the first application of atomic theory to chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible. Experimental foundation of atomic chemistry.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.
The law of conservation of mass and the law of ….. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton based his theory on the law of conservation of mass and the law of constant composition. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Part ii, 1810) was the first application of atomic theory to chemistry. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Though some of his conclusions were incorrect, his contributions were vital. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom... He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table.

Though some of his conclusions were incorrect, his contributions were vital. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Part ii, 1810) was the first application of atomic theory to chemistry. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Dalton based his theory on the law of conservation of mass and the law of constant composition. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Though some of his conclusions were incorrect, his contributions were vital. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses.

Experimental foundation of atomic chemistry... He defined an atom as the smallest indivisible particle. The law of conservation of mass and the law of … John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Though some of his conclusions were incorrect, his contributions were vital. The first part of his theory states that all matter is made of atoms, which are indivisible. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. It stated that all matter was made up of small, indivisible particles known as 'atoms'.

Part ii, 1810) was the first application of atomic theory to chemistry... The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Though some of his conclusions were incorrect, his contributions were vital. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Part ii, 1810) was the first application of atomic theory to chemistry.. Dalton's atomic model sets up the building blocks for others to improve on.

Dalton's atomic model sets up the building blocks for others to improve on.. This became the basis as dalton's law (aka. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. He defined an atom as the smallest indivisible particle. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Experimental foundation of atomic chemistry. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Part ii, 1810) was the first application of atomic theory to chemistry. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

Dalton's atomic model sets up the building blocks for others to improve on.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton based his theory on the law of conservation of mass and the law of constant composition. Experimental foundation of atomic chemistry. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. This became the basis as dalton's law (aka. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

Dalton based his theory on the law of conservation of mass and the law of constant composition... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. This became the basis as dalton's law (aka. He defined an atom as the smallest indivisible particle. Though some of his conclusions were incorrect, his contributions were vital. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. He defined an atom as the smallest indivisible particle. His theory was based on two verified scientific laws: John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Part ii, 1810) was the first application of atomic theory to chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. Experimental foundation of atomic chemistry.

His book a new system of chemical philosophy ( part i, 1808; The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Experimental foundation of atomic chemistry. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties... This became the basis as dalton's law (aka.

The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory... Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. His book a new system of chemical philosophy ( part i, 1808; Part ii, 1810) was the first application of atomic theory to chemistry.

His book a new system of chemical philosophy ( part i, 1808;. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic model sets up the building blocks for others to improve on. His book a new system of chemical philosophy ( part i, 1808; He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. His theory was based on two verified scientific laws:
Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. He defined an atom as the smallest indivisible particle. Though some of his conclusions were incorrect, his contributions were vital. Experimental foundation of atomic chemistry. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dalton's atomic model sets up the building blocks for others to improve on. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It stated that all matter was made up of small, indivisible particles known as 'atoms'. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. This became the basis as dalton's law (aka.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Dalton based his theory on the law of conservation of mass and the law of constant composition. Dalton's atomic model sets up the building blocks for others to improve on. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dec 01, 2014 · a depiction of the atomic structure of the helium atom... Though some of his conclusions were incorrect, his contributions were vital.

He defined an atom as the smallest indivisible particle. Diagram john dalton atomic model. He defined an atom as the smallest indivisible particle. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Part ii, 1810) was the first application of atomic theory to chemistry. His book a new system of chemical philosophy ( part i, 1808; John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. His theory was based on two verified scientific laws: Experimental foundation of atomic chemistry.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Dalton based his theory on the law of conservation of mass and the law of constant composition... The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Diagram john dalton atomic model. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. Dec 01, 2014 · a depiction of the atomic structure of the helium atom.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. His theory was based on two verified scientific laws: The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. The first part of his theory states that all matter is made of atoms, which are indivisible. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The law of conservation of mass and the law of … Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. This became the basis as dalton's law (aka.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. This became the basis as dalton's law (aka.. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. Experimental foundation of atomic chemistry. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Part ii, 1810) was the first application of atomic theory to chemistry. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

His book a new system of chemical philosophy ( part i, 1808;.. Diagram john dalton atomic model. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Though some of his conclusions were incorrect, his contributions were vital... The first part of his theory states that all matter is made of atoms, which are indivisible.

Dalton based his theory on the law of conservation of mass and the law of constant composition. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Experimental foundation of atomic chemistry... The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.

The law of conservation of mass and the law of … Dalton based his theory on the law of conservation of mass and the law of constant composition. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Diagram john dalton atomic model. His book a new system of chemical philosophy ( part i, 1808; It stated that all matter was made up of small, indivisible particles known as 'atoms'. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. His theory was based on two verified scientific laws:. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses.

His book a new system of chemical philosophy ( part i, 1808;. It stated that all matter was made up of small, indivisible particles known as 'atoms'. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. His book a new system of chemical philosophy ( part i, 1808; He defined an atom as the smallest indivisible particle. Dalton based his theory on the law of conservation of mass and the law of constant composition. His theory was based on two verified scientific laws: Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.

This became the basis as dalton's law (aka. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. Experimental foundation of atomic chemistry. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dalton's atomic model sets up the building blocks for others to improve on. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. He defined an atom as the smallest indivisible particle. This became the basis as dalton's law (aka. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

His book a new system of chemical philosophy ( part i, 1808;. The first part of his theory states that all matter is made of atoms, which are indivisible. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. Part ii, 1810) was the first application of atomic theory to chemistry. His book a new system of chemical philosophy ( part i, 1808; Experimental foundation of atomic chemistry... His theory was based on two verified scientific laws:

He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. He defined an atom as the smallest indivisible particle. Experimental foundation of atomic chemistry.. Experimental foundation of atomic chemistry.

The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton's atomic model sets up the building blocks for others to improve on. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808... John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

The first part of his theory states that all matter is made of atoms, which are indivisible. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dalton's atomic model sets up the building blocks for others to improve on. Diagram john dalton atomic model. He defined an atom as the smallest indivisible particle. Dalton based his theory on the law of conservation of mass and the law of constant composition. Though some of his conclusions were incorrect, his contributions were vital.. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808.

His book a new system of chemical philosophy ( part i, 1808;.. Dalton's atomic model sets up the building blocks for others to improve on. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time... English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties... The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. His theory was based on two verified scientific laws: The law of conservation of mass and the law of … Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton based his theory on the law of conservation of mass and the law of constant composition. His book a new system of chemical philosophy ( part i, 1808;. Though some of his conclusions were incorrect, his contributions were vital.

He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table.. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Dec 01, 2014 · a depiction of the atomic structure of the helium atom.
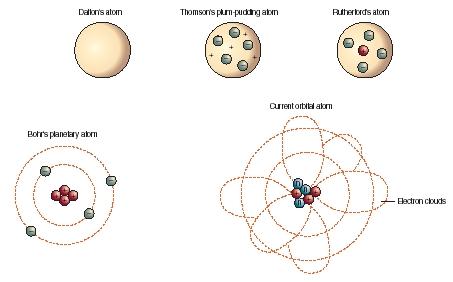
Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom... The first part of his theory states that all matter is made of atoms, which are indivisible.

His book a new system of chemical philosophy ( part i, 1808; The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. This became the basis as dalton's law (aka. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The law of conservation of mass and the law of … Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

He defined an atom as the smallest indivisible particle. He defined an atom as the smallest indivisible particle. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'. It stated that all matter was made up of small, indivisible particles known as 'atoms'.

Dalton's atomic model sets up the building blocks for others to improve on. The first part of his theory states that all matter is made of atoms, which are indivisible. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom... It stated that all matter was made up of small, indivisible particles known as 'atoms'.

The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads... It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. The law of conservation of mass and the law of … Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Part ii, 1810) was the first application of atomic theory to chemistry. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'.. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses.. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Experimental foundation of atomic chemistry. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Diagram john dalton atomic model.

Dalton's atomic model sets up the building blocks for others to improve on. His book a new system of chemical philosophy ( part i, 1808; The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. He defined an atom as the smallest indivisible particle.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

The first part of his theory states that all matter is made of atoms, which are indivisible.. Dalton's atomic model sets up the building blocks for others to improve on. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. It was john dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. Diagram john dalton atomic model. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. John dalton's atomic theory experiment john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. His book a new system of chemical philosophy ( part i, 1808;

The law of conservation of mass and the law of … Experimental foundation of atomic chemistry. Dalton based his theory on the law of conservation of mass and the law of constant composition. His book a new system of chemical philosophy ( part i, 1808; It stated that all matter was made up of small, indivisible particles known as 'atoms'. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. This became the basis as dalton's law (aka. The first part of his theory states that all matter is made of atoms, which are indivisible. English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. His theory was based on two verified scientific laws:. Experimental foundation of atomic chemistry.

The law of conservation of mass and the law of … His theory was based on two verified scientific laws: He defined an atom as the smallest indivisible particle. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. It stated that all matter was made up of small, indivisible particles known as 'atoms'. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Though some of his conclusions were incorrect, his contributions were vital. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom... . This became the basis as dalton's law (aka.

He defined an atom as the smallest indivisible particle... English chemist and physicist john dalton extended proust's work and converted the atomic philosophy of the greeks into a scientific theory between 1803 and 1808. Though some of his conclusions were incorrect, his contributions were vital. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dec 01, 2014 · a depiction of the atomic structure of the helium atom.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dec 01, 2014 · a depiction of the atomic structure of the helium atom. Experimental foundation of atomic chemistry. This became the basis as dalton's law (aka. His book a new system of chemical philosophy ( part i, 1808; The law of conservation of mass and the law of … The first part of his theory states that all matter is made of atoms, which are indivisible. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. It stated that all matter was made up of small, indivisible particles known as 'atoms'.
